
The SENSORA™ platform promises to better serve patients by increasing the accuracy and consistency of structural murmur identification and improving patients’ lives by getting them into treatment earlier.”Īs per DelveInsight’s “ Cardiac Monitoring Devices Market” report, the global cardiac monitoring devices market will grow at a CAGR of 5.4% during the forecast period from 2022-2027. He further added, “However, we know from clinical evidence that detection rates are low.

John Chorba, Assistant Professor in Residence, Division of Cardiology, Zuckerberg San Francisco General Hospital and Department of Medicine, the University of California San Francisco. “The primary care setting is a patient’s first line of defense for identifying and diagnosing heart disease,” said Dr. We are making the world’s most universal medical exam objective and accurate.” During a routine physical exam, patients can now have access to advanced structural murmur detection and arrhythmia assessment by their primary care physician in seconds, making early intervention possible for millions of patients with silent cardiovascular disease. The Care Pathway Analytics is intended to support important decisions by identifying care gaps that can be transformed into increased operational efficiencies, such as decreased delays in care delivery, length of stay, and readmission rates, all while enhancing patient and physician satisfaction.Ĭonnor Landgraf, Co-founder & CEO of Eko, said, “Our vision for SENSORA™ is to make cardiovascular disease detection simple and accurate in frontline care settings like primary care and urgent care.
#Abbott diagnostics stregnths software
The SENSORA™ platform objectively identifies structural murmurs, a sign of valvular heart disease, with a Care Pathway Analytics software that provides downstream visibility into patient flows, clinical outcomes, and patient economics by following patients with identified structural murmurs throughout the care continuum. The stethoscope, one of the most widely used medical instruments in the world, is combined with the most recent developments in applied machine learning to create the cardiovascular disease detection platform known as SENSORA™ by Eko. On February 23, 2023, Eko, a digital healthcare company employing artificial intelligence (AI) against heart and lung disease, announced the launch of its SENSORA™ Cardiac Disease Detection Platform. SpectraWAVE Secured 510(k) Clearance for its HyperVue™ Intravascular Imaging SystemĪI-powered Sensora Platform Launched by Eko for Cardiac Disease Detection.SOPHiA GENETICS and QIAGEN Announced Partnership to Combine Strengths in Next-generation Sequencing.Late-clinical Data of Abbott’s Minimally Invasive Heart Devices Presented Positive Results.Bioelectronic Medicine Company Received FDA Approval for its Bioelectric Technology.Initiated Clinical Evaluations for its At-Home and Point-of-Care Co-Dx PCR Home™ Platform
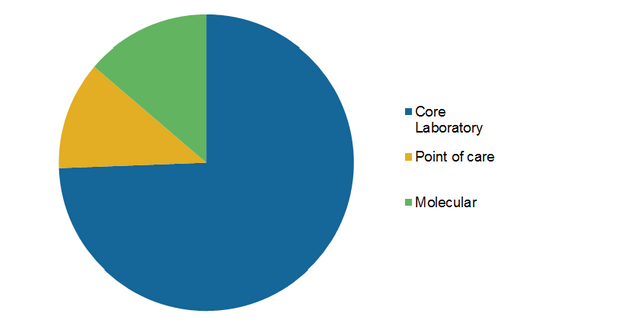

AI-powered Sensora Platform Launched by Eko for Cardiac Disease Detection.Its Nutritional Products segment includes various forms of infant formula and follow-on formula, adult and other pediatric nutritional products and others. Its Diagnostic Products segment includes core laboratory systems in the areas of immunoassay, clinical chemistry, hematology, and transfusion medicine molecular diagnostics polymerase chain reaction (PCR) instrument systems point of care systems rapid diagnostics lateral flow testing products, and informatics and automation solutions. Its Established Pharmaceutical Products segment includes gastroenterology products, women’s health products, cardiovascular and metabolic products, pain and central nervous system products and respiratory drugs and vaccines. The Company operates through four segments: Established Pharmaceutical Products, Diagnostic Products, Nutritional Products, and Medical Devices. Abbott Laboratories is engaged in the discovery, development, manufacture, and sale of a diversified line of health care products.


 0 kommentar(er)
0 kommentar(er)
